Background: Multiple myeloma (MM) patients with chromosome 1q gain (1q+) are clinically and biologically heterogeneous. The underlying molecular mechanisms are still under investigation, while identification of targets for effective therapy of this subgroup of MM patients is urgently needed. We aimed to investigate the clinical significance and the regulatory mechanisms of insulin-like growth factor 2 messenger RNA (mRNA) binding proteins 1 (IGF2BP1), a N6-methyladenosine (m6A) reader, in MM patients with 1q+.
Methods: Comprehensive in silico analyses of existed public datasets (MMRF CoMMpass study) and in-house generated high-throughput sequencing datasets (RNA-sequencing, methylated RNA immunoprecipitation sequencing, and crosslinking immunoprecipitation sequencing), along with routine molecular and cellular in vitro assays, as well as in vivo animal model studies, were conducted.
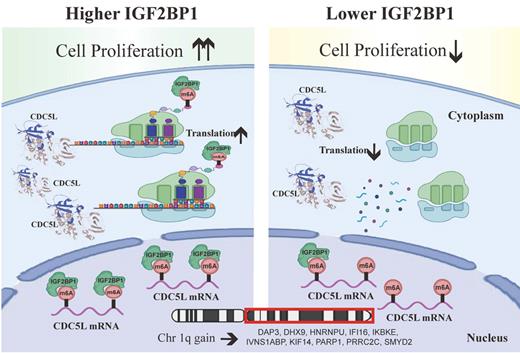
Results: IGF2BP1 displays a high expression level in MM patients with 1q+ and its high expression predicts poor prognosis in these patients. IGF2BP1 overexpression promoted cell proliferation and G1-to-S phase transition of the cell cycle in H929 cells. Combinatory analyses of MeRIP-seq, RNA-seq, CLIP-seq and patients' survival data identified CDC5L as a target of IGF2BP1, which can bind the m6A sites of CDC5L mRNA to upregulate its protein abundance. Higher CDC5L expression also predicted worse prognosis of MM patients with 1q+. Moreover, both knockdown and mutation of CDC5L attenuated the pro-proliferative effect of IGF2BP1. Furthermore, IGF2BP1 inhibitor BTYNB effectively inhibited CDC5L expression in MM cells with 1q+, and suppressed the proliferation of these cells in vitro and in vivo.
Conclusions: IGF2BP1 acts as a post-transcriptional enhancer of CDC5L in an m6A manner to promote the proliferation of MM cells with 1q+. Our work identified a novel IGF2BP1-CDC5L axis and provided new insight in developing targeted therapeutics for MM patients with 1q+.
Disclosures
No relevant conflicts of interest to declare.